
Each electron was forced to hold the little ground he or she had left at any cost forgotten were the days where electrons were bound by society to stay the customary distance away from one another. Overcrowding began to run rampant, and the electrons degenerated into survival mode. Electrons from all around were forced to move into the estates when their suburban lifestyle became untenable. To understand electron degeneracy pressure in terms of the Pauli Exclusion Principle, let us consider a parable of overdevelopment in the recently built "Micro-Estates" gated community for electrons of evolved stars.Īfter the great collapse of the core corporation where millions of electrons were employees, conditions in the once envied Micro-Estates deteriorated. While it is true that most stars in our galaxy are about the same size as our sun and will end their lives enigmatically as burnt cinders, this does not necessarily spell the end of every low mass star's evolution.Īs we noted before in section 3.2 of the course wiki, a white dwarf is supported against gravity by electron degeneracy pressure. We have discovered that the death of a massive star is not the only way to make a supernova. White Dwarf Stars: The Evolution Continues To understand why these stars best explain the homogeneities in Type Ia supernovae, we must first look more closely at the conditions inside of a white dwarf. As it turns out, surprisingly perhaps, the likely culprit for these supernovae is the lowly white dwarf star. These homogeneities were the first indication that there seems to be a unique process or set of conditions that lead to Type Ia supernovae. Light curves and spectra from Type Ia supernovae are remarkably homogeneous, especially when compared to the other types and sub-types of supernovae. The figure below shows sample light curves from various types of supernovae. Type IIp supernovae are given the subgroup p, which stands for "plateau", since their light curves maintain a brightness close to that of their maximum brightness for a relatively long period of time, compared to others that fall off more rapidly after the time of maximum light). Light curves are used to subdivide the Type II supernovae based upon how their brightness changes with time (e.g. Unlike the light curve you saw with GK-Per, a supernova light curve is not periodic since the explosion only occurs once. The light curves among the different Type I supernovae are much more homogeneous than the light curves among Type II supernovae.Īnother key piece of information astronomers use to classify supernovae is their light curves. Type Ib and Type Ic are characterized by the presence or absence of a helium line around 5876 Å (vertical purple. The spectra of a Type Ia supernova contain a distinct silicon absorption line around 6150 Å (vertical orange line as seen in the figure) this line is unique among Type I supernovae and so defines the subgroup-a of the Type I supernovae. We distinguish three sub-types of Type I supernovae: Type Ia, Type Ib, and Type Ic.

We believe that all of the Type II supernova result from the collapse of a massive star's core that leave behind a compact stellar remnant in the form of a neutron star or black hole. In the figure, the spectral signatures are seen as absorption lines (a dip in brightness from the continuum at a wavelength that corresponds to a specific electronic transition in a specific element).
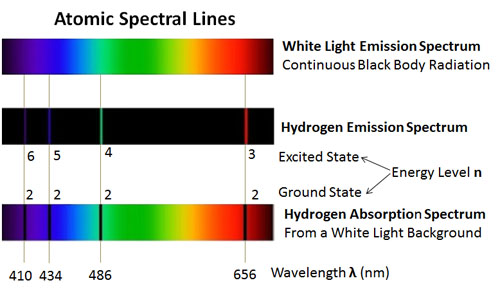
The defining characteristic of a Type I supernova is a lack of hydrogen (vertical teal lines near maximum light as shown in the figure below at 6563Å) in their spectra, whereas Type II supernovae do show spectral lines of hydrogen. Supernova are fundamentally classified by their atomic spectra into two groups: Type I and Type II, examples of which are seen in optical light in the figure below (the x-axis of the plot is in angstroms (Å), which are defined as 1Å=1.0×10 -10m=0.1nm, while the y-axis is a measure of the brightness at various wavelength observed with a spectrograph).


 0 kommentar(er)
0 kommentar(er)
